CRISPR: The genetic editing tool that holds promise & peril
Chinese researcher He Jiankui has claimed that he used CRISPR to produce the world’s first “designer babies”. On Wednesday, he said a second pregnancy was underway. A look at the genetic editing system that may change our relationship with genetics for better, worse or both.
Why is CRISPR in the news?
He Jiankui has claimed that he produced the world’s first genetically-edited babies. Jiankui said he used CRISPR—Clustered Regularly Interspaced Short Palindromic Repeats—to alter the genes of a pair of twins while they were embryos to make the babies resistant to HIV, the virus that causes AIDS. He said a possible second pregnancy had resulted from his project. The announcements prompted mostly criticism from other experts in the field. Using CRISPR to make changes to embryos and germline cells—sperm, eggs and zygotes—is contentious as the modifications are passed to progeny.
Can genetic editing prevent serious diseases?
In 2017, a US science and medicine advisory group decided to support research using technologies such as CRISPR to modify human embryos for prevention of serious diseases and disabilities. In experiments with human cells, CRISPR has been used to repair a mutation that causes blindness and correct the defect responsible for cystic fibrosis. The first human trial began in China in 2016 using CRISPR-modified T-cells to treat lung cancer patients. Two studies published in mid-2018 found that cells edited by CRISPR have the potential to seed tumours, raising the risk they would trigger cancer, but the link is still under investigation.
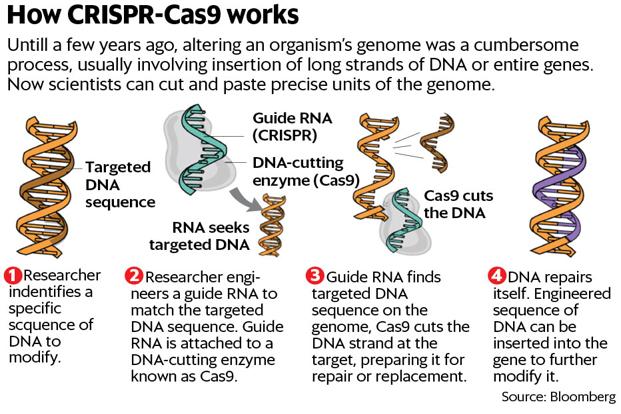
What is CRISPR-Cas9?
CRISPR-Cas9 is a rudimentary immune system first noticed in bacteria nearly 30 years ago. They are sequences of genetic code broken up by remnants of genes from past invaders.
How is CRISPR used?
In 2012, researchers at the University of California, Berkeley, published a paper on making molecular “guides” that allow CRISPR to skim along DNA, targeting exactly the right spot to make a slice. Then, scientists at Broad Institute said they’d adapted it for use in human cells. A researcher with basic skills and equipment worth a few thousand dollars can employ CRISPR. The gene-editing system isn’t perfect yet: it makes unintended cuts in DNA as often as 60% of the time in some applications, with effects unknown.
What’s the argument against using it?
Decisions about whether to use CRISPR to treat people who are already sick could be made through traditional consideration of risks and benefits, once they are better understood. The potential to do good is enormous: eliminating a genetic disease from a family forever. But if something goes wrong, the consequences are potentially eternal, too, affecting future generations who could not give prior consent. Some scientists worry that germline editing would invite enhancements of babies for non-medical reasons.
…………………………………………………
Scientists in India are deeply divided over the implications of the recent shock announcement about gene-edited “designer” babies made by a Chinese scientist. The responses range from disbelief about the research to potential implications for next-generation science. On Monday, He Jiankui, a scientist from China, presented his findings at a genome summit in Hong Kong claiming to have created the world’s first gene-edited twins. Jiankui edited a gene in the embryo and implanted it in the mother’s womb to make the babies resistant to HIV infection. The twin girls were delivered last month, he said.
“He created a next-generation baby, which almost falls in the purview of designer babies. It sets a wrong precedent. Creating a child with specific traits or deciding how the next generation will be could open the technology for potential misuse by those who have the tools, funds and necessary resources,” said senior scientist Debojyoti Chakroborty from CSIR-Institute of Genomics and Integrative Biology (IGIB), Delhi, “It was definitely not the necessity at the moment,” he added.
Several research laboratories across India are using gene-editing tool CRISPR to correct mutations in genetic disorders like sickle cell anaemia and haemophilia by isolating embryonic stem cells derived from patients, trying to establish preclinical studies and examining if these can be used for therapy. Some are doing basic research on CRISPR, while others are engaged in identifying genes in plant and animal genomes after knockout or knock-in of a gene to study their function and impact.
Ever since its discovery in 2012, consensus has prevailed among international scientists not to use CRISPR for editing embryos, until it is proven to be completely safe. However, research by the Chinese scientist, which is yet to be published in any journal, or ‘peer-reviewed’, has set the alarm bells ringing on its future consequences, especially when the research in the domain is on at an exponential rate.
Girish Sahni, former director-general, Council of Scientific and Industrial Research (CSIR), who is credited with developing India’s first indigenous clot-buster drug for cardiovascular diseases, said, “The technology is fairly developed to reap awards. But the focus should be on its monitoring and ensuring strict guidelines to regulate its use.”
That said, Sahni is excited about the potential. “Gene-editing is a powerful tool. India has already lost precious time and it is yet to make any breakthrough in this domain. We should immediately work out modalities by which the research in this area can be conducted, but it should be tied to societal needs, especially agriculture, where it has the maximum scope. We can improve the quality of livestock and agriculture products.”
Plant geneticist, Imran Siddiqui from the Centre for Cellular and Molecular Biology (CCMB), says the technology offers a big advantage. “If you mutate one gene, it does not cause any evident change in the plant or organism, because the function of that one gene is taken over by a related gene. But, CRISPR offers the advantage of targeting multiple members of the gene family for mutations in just one go, to show visible consequences,” he said.
The underlying concerns are more on the validity of the current research, risk assessment and safety, and ethics as it is believed that the edited embryo could become more vulnerable to other infections. “It is shrouded in mystery. It will take several years to understand how safe it is,” said Chakroborty.
Scientists also contend that there are more pressing disorders that could have been taken up for research.
“It is indeed the ‘therapy of the future’, be it cancer biology and neurodegenerative diseases. If we can isolate the defective genes, it opens up huge applications for disease-control. But, there is still a long way to go. We are taking the first few steps,” said Amitava Sengupta, senior scientist from CSIR-Indian Institute of Chemical Biology (IICB), Kolkata